Magnetic Resonance Imaging (MRI) Safety Program
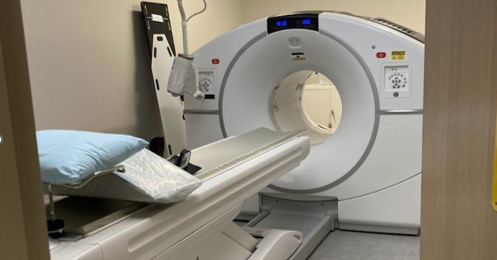
Magnetic Resonance Imaging (MRI) uses radio waves and a strong magnetic field to provide detailed pictures of internal organs and tissues. Using specialized equipment, MRI generates high resolution images that have the ability to identify many diseases in the brain, spine, musculoskeletal system and abdominal and pelvic organs. MRI is the most advanced diagnostic imaging tool in the evaluation of many medical conditions. MRI does not use ionizing radiation (X-rays). MRI machines use magnets and radio waves to make images of the body.
MRI Safety Risks
There are potential risks in the magnetic resonance (MR) environment, not only for the patient 1,2
but also for the attending health care professionals, accompanying family members, and others, including security officers, housekeeping personnel, firefighters, police, etc, who may encounter the magnetic fields and other energy sources associated with MR scanners. The UTRGV MRI Safety Manual contains guidelines that apply to clinical diagnostic imaging, research, and atypical MR settings (eg, linear accelerator MR, interventional MR, etc) and encompass information for patients, research subjects, and health care personnel.
Magnetic Field Risk: The static magnetic field of the MRI system is exceptionally strong. A 3.0 T magnet generates a magnetic that is approximately 60,000 greater than the earth's natural magnetic field. In such an environment ferromagnetic metal objects can become airborne as projectiles. Small objects such as paper clips and hairpins have a terminal velocity of 80 mph when pulled into a 3.0 T magnet and therefore pose a serious risk to the patient and anyone else in the scan room. The force with which projectiles are pulled toward a magnetic field is proportional to the mass of the object and distance from the magnet. Even surgical tools such as hemostats, scissors and clamps, although made of a material known as surgical stainless steel, are strongly attracted to the main magnetic field. Oxygen tanks, gurneys, floor buffing machines, and construction tools are highly magnetic and should never be brought into the scan room.
Consumer products such as pagers, cell phones, cameras and analog watches may be damaged by the magnetic field. Pacemakers may be reprogrammed or turned off by the magnetic field. The magnet field erases credit cards with magnetic strips. Patients with ferrous intra-cranial vascular clips may be at risk due to the possible movement of the clip. People with pacemakers cannot be scanned or even go near the scanner because the magnet can cause the pacemaker to malfunction.
Prior to allowing a patient or support staff member into ZONE III and the scan room, he or she is thoroughly screened for metal objects -- and not just external objects.
Radio-frequency (RF) Field Risk
The radio-frequency field may induce currents in wires that are adjacent or on the patient, causing skin burns. It may induce currents in intra-cardiac leads, resulting in inadvertent cardiac pacing. Prolonged imaging may cause the patient's core body temperature to rise. In practice, significant patient heating is only encountered in infants.
Due to the risk of RF current induced thermal burns:
- To minimize the risk of synthetic fibers in clothes acting as a current inducer, all patients having a MR exam must be changed into hospital provided clothing (gowns) prior to imaging.
- All patients having a MR exam must be padded during imaging in accordance to manufacturer guidelines to minimize skin to skin, and skin to magnet bore contact.
- All patients must be provided a working alert device (squeeze ball), to able to communicate with the MRI technologist during imaging when in distress.
Cryogen Risk: During a planned or accidental shutdown of the magnetic field (aka "quench"), the liquid Helium in the magnet turns into gas and may escape into the scan room displacing the oxygen in the room leading to asphyxia.
Biological Effects Due to Magnetic Field: For the static magnetic fields currently used in MRI up to 2 Tesla, there are no known biological effects. The majority of studies show no effects on cell growth and morphology. Data accumulated by the National Institute for Occupational Safety, the World Health Organization, and the US State Department show no increased risk for leukemia or other cancer. Some reversible biological effects have been observed on human subjects exposed to 2.0 T and above. These effects include fatigue, headaches, hypotension, and irritability.
Access Restriction
This following section summarizes the different zones of a UTRGV MR suite and points out specific safety issues of greatest concern. At UTRGV, each MRI site is divided into 4 safety zones based on the American College of Radiology guidelines:
Zone 1:
General public area outside the MR environment. This area is the reception and waiting areas.
Zone 2:
Area between Zone 1 (Public Access) and the strictly controlled Zone 3 (Control Room) and Zone 4 (Magnet). This is the area just outside of the restricted area Zone 3. This is the area of travel that patients are brought into their procedure.
Zone 3:
Control Room. All access to Zone 3 is to be strictly restricted with access to regions within it controlled by and entirely under the supervision of MR personnel. This zone is restricted from general public access by a reliable restricting method that can differentiate between MR and non-MR personnel.
Zone 4:
Magnet Room. No individual is allowed in the scan room without being supervised by trained MRI personnel. The scan room door is always locked when unattended. Only MR compatible equipment approved may be brought into Zone 4. The MR technologists must be able to directly observe and control via line of sight the entrances or access to Zone 4 from their normal positions when stationed at their desks in the scan control Room.
MRI Safety Guidelines
MRI safety guidelines are established by URGV UTRGV and apply to all clinical and research MRI systems operated by the UTRGV School of Medicine . MRI procedures performed at UTRGV will be conducted in accordance with established protocols and in reference to the American College of Radiology Guidelines. Appropriate precautions will be followed to ensure safety of all patients, personnel and equipment. Safety policies and procedures are evaluated on a annual basis by the MRI Safety Officer.
MRI Safety Program Documents
UTRGV MRI Patient Safety Screening - Poster 1
UTRGV MRI Patient Safety Screening - Poster 2
UTRGV MRI Patient Screening Form
Training
MRI training is mandated for all personnel who have access Zone 2. There are 2 levels of MR Personnel, as described below.
Level 1 MR Personnel: Individuals who have passed the facility’s MR safety educational requirements (as defined by the facility’s MRMD) to ensure that they would not constitute a danger to themselves or others in the MR environment will henceforth be referred to as Level 1.
Level 2 MR Personnel: Those who have been more extensively trained and educated in the broader aspects of MR safety issues, including but not limited to issues related to the potential for RF-related thermal loading or burns and direct neuromuscular excitation from rapidly changing gradients, will henceforth be referred to as Level 2 MR Personnel.
Training is provided on a needed basis. Contact the ESHRM for training at (956) 665-2903 or email ehsrm@utrgv.edu.
