Getting Started
Getting Started
The first part of the Research Start-Up Process can be broken down into steps.
- Step 1: Complete the Required Training
- Step 2: Submit Your Study into ARGO/START
- Step 2b: Coverage Analysis and Building a Research Budget
The next parts of the Research Start-Up Process must to be completed but can be done at the same time, in no particular order. Click on each part below for more information.
Step 1: Complete the Required Training
Anyone conducting, or involved in the conduct, of research must complete training. Different categories of research and different roles within the research team may require different training modules. However, every member of the research team must have the appropriate training.
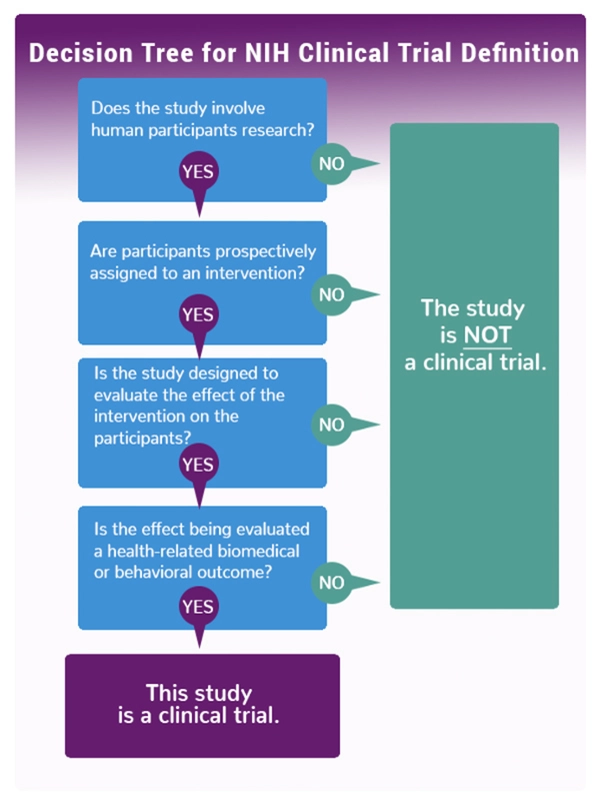
Research that is considered a Clinical Trial has very specific training requirements. Some types of studies involving human subjects may be considered a clinical trial by regulatory definition, UTRGV Institutional Guidelines and/or by funder/sponsor definitions. UTRGV IRB can help you understand if your research is considered a clinical trial.
To access the instructions for registration and completion of the required training at UTRGV, visit the IRB Trainings | UTRGV.
Step 2: Submit Your Study Into ARGO/START
Automated Research and Grant Organizer (ARGO) is a platform for streamlining processes with all sponsored projects.
Within ARGO there is a tool specifically designed for clinical research – the Study Type Assessment and Regulatory Tracking (START) form. For more information, email clinicalresearch@utrgv.edu.
WHAT TYPE OF STUDIES NEED TO BE ENTERED INTO START?
- The START Form is required for any prospective clinical research, including Observational and Registry studies, that are based at UTRGV, UT Health RGV, or any UTRGV facility (main campus or any outpatient clinic), AND involves any interaction with the human participant after consent is obtained. **UTRGV uses the NIH definitions for clinical research and clinical trials.**
If your study meets the above criteria, the START form is where you will submit all the documents and information for your study in order for the Office of Clinical Research to determine how to help you move forward.
START User Guides and FAQs:
- Data Project/Requesting Research Data User Guide and FAQs
- Study Type Assessment and Regulatory Tracking (START) Form User Guide and FAQs
- Clinical Research FAQs
If you have any questions about your submission in ARGO/START, contact the Office of Clinical Research at clinicalresearch@utrgv.edu.
Step 2b: Coverage Analysis and Building a Research Budget
Once you have submitted all study documents into START, the Office of Clinical Research will determine if your study needs a coverage analysis.
A coverage analysis (CA) or Medicare Coverage Analysis (MCA) is a uniform methodology of analyzing the items and services provided in a clinical research study, i.e., a comprehensive review of protocol documents that helps identify the appropriate payor (sponsor or third-party) for each study service, assessment of the protocol driven items and procedures that can be billed to either insurance, as routine cost, or as costs billable to the study sponsors.
- Minimize potential errors in research billing: It reduces the risk of improper billing. The False Claims Act targeted at “fraud” violations may be triggered by inaccurate billing. By sending bills to agencies inappropriately, the institution and the individuals risk paying civil and criminal penalties and exclusion from participation in future research. If the Office of the Inspector General discovers improper billing, they can exclude institutions from Medicare or Medicaid or other federal programs.
- Provide a template for budget development and negotiation: It can help justify payments to the institutions, strengthen the budget development process and further create efficiency in budget negotiations. It maximizes recovery of study related cost by getting reimbursed for items and services not standard of care.
- Prevent patient Dissatisfaction and undue financial burden: There can be “double billing” for billing CMS or third-party payers for items that have already been paid by a sponsor. You can have unhappy patients who are under financial burdens as they are receiving these incorrect or additional bills on services, which ultimately affects the reputation of the institution. This may be an indicator of billing problems and process problems. Therefore, there is a need to assess and evaluate trends and to recognize these issues by getting the sequence right from the Medicare Coverage Analysis assessment at the beginning of a study.
- Promote and Streamline Billing compliance: Medicare Coverage Analysis helps coordinate billing and administrative activities and downstream communication to the billing staff and other member of the research team, thereby reducing the number of hours spent reviewing charges and providing the road map. It streamlines billing compliance.
It is a UTRGV policy (FM-701) for all studies that generate a charge to either the patient/payor or research account to have a coverage analysis.
- The Office of Clinical Research completes the coverage analysis for each study.
- Make sure you have submitted ALL study documents available in. Protocol, consent, manuals, questionnaires, budgets, etc. submitted within the START form.
- Coverage analysis cannot be completed without all study documentation. This includes, but is not limited to:
- Protocol
- Informed Consent(s)
- All study manuals and guidance documents
- Study budget – even if not finalized
- The START form must also include the name of the Principal Investigator at UTRGV, study title, sponsor (if applicable).
- If the IRB number has already been received, include it in the free text section within the START form.
- If the application to the IRB is still in progress, include the date submitted to the IRB in lieu of the IRB number.
- The Coverage Analyst will respond to the study team within three (3) business days with an estimated timeline for completion.
- Respond promptly to any requests for information from the Coverage Analyst or the Office of Clinical Research.
- Once completed, the coverage analysis document will be given to the PI for review and signature. It is the responsibility of the PI to bring forward any questions, change requests, or discrepancies to the coverage analysis team.
- Once all documentation is agreed upon, the PI will sign the final document(s) and return them to the coverage analysis team. These documents will be shared with the budgets and contracts teams.
- If there are any questions along the way, contact clinicalresearch@utrgv.edu.
*Under Construction - Content Coming Soon.
If your study is Investigator Initiated at UTRGV, visit the pre-award process page or contact sponpro@utrgv.edu for more information.
If your study is sponsored or funded by an outside company or institution, you should have a contact person from that company or institution that can provide your budget.
If you are unsure, contact clinicalresearch@utrgv.edu for guidance.
Research Contracts
There are many reasons a research study would require a contract and many different types of contracts to address the specific needs of each study. Here are some of the most commonly used contracts in clinical research.
Material Transfer Agreement (MTA) - a contract that governs the transfer of tangible research materials between two organizations, when the recipient intends to use it for his or her own research purposes. The MTA defines the rights of the provider and the recipient with respect to the materials and any derivatives, such as intellectual property. For example, when requesting biospecimens from another institution an MTA would need to be established between UTRGV and the other institution.
Data Transfer and Use Agreement (DTUA/DUA) – a contract between the entity that owns access to a data source, typically a dataset or database, and a secondary entity that will receive the data, or a subset of it, for reuse in clinical research. For example, when requesting access to the electronic medical records of another institution a DTUA would need to be established between UTRGV and the other institution.
Clinical Trial Agreement (CTA) – an agreement between UTRGV and an industry sponsor (typically a pharmaceutical or device company) to conduct a clinical study in which the protocol is written and provided by the industry sponsor. When a clinical trial agreement is in place, separate agreements are not required for use of the vendors required by the sponsor during the conduct of the study.
Sponsored Research Agreement (SRA) – an agreement between UTRGV and a sponsor to conduct a non-clinical or basic science research study when the protocol is written by a UTRGV faculty member(s) and funded by the sponsor.
The Office of Research Contracts and Industry Agreements is responsible for determining what contracts will be required for your study. They partner with the research team and the Office of Clinical Research to ensure nothing is overlooked. Contact rcia@utrgv.edu for questions or information about research contracts.
Regulatory Requirements and Resources
Institutional Review Board (IRB)
No research may be conducted at UTRGV without an approval letter from the UTRGV Institutional Review Board. Even if your study uses a different or central IRB, the UTRGV IRB must approve the reliance on the other IRB. Follow the guidance found on the UTRGV IRB website for explicit instructions on how to submit your research. For more information visit the Institutional Review Board (IRB) | UTRGV.
Institutional Biosafety Committee (IBC)
Research involving viral vectors, genetically modified materials, plasmid, recombinant DNA (rDNA), messenger RNA (mRNA), synthetic nucleic acids, and/or vaccines must also undergo Institutional Biosafety Committee review. You may use UTRGV IBC or an outside/commercial IBC. If your study requires IBC review, you must have the approval in place before you begin. For more information visit the Institutional Biosafety Committee (IBC) | UTRGV.
Institutional Animal Care and Use Committee (IACUC)
For research involving animals, please visit Laboratory Animal Resources (LAR) | UTRGV.
The US Food and Drug Administration (FDA) is the government agency that requires registration of clinical trials. The FDA requires mandatory registration and results reporting for certain clinical trials of drugs, biologics, and devices of all applicable clinical trials. The legislation coupled with the Final Rule for Clinical Trials Registration and Results Information Submission creates the regulatory requirements and procedures for ClinicalTrials.gov. See UTRGV PM-306 and UTRGV PM-307 for more information.
The Office of Clinical Research can help you determine if your study needs registration and reporting within ClinicalTrials.gov. If required, the OCR can also help you with the completion of those tasks. For further questions, contact clinicalresearch@utrgv.edu.
Florence is an electronic platform designed to store regulatory files in a safe and compliant manner. Among other things, Florence facilitates electronic signatures on regulatory documents and remote monitoring and auditing of study files.
All clinical trials at UTRGV that have not yet enrolled any human subjects prior to September 1 st, 2024 are required to store their regulatory files within Florence. Studies that have enrolled human subjects before September 1 st, 2024, may use Florence, if desired.
Florence Required
- All new clinical trials
- Open clinical trials that have not yet enrolled any human subjects
Florence Recommended
- Open clinical trials that have already enrolled human subjects
- Human subjects research that does not meet the NIH definition of a clinical trial
Florence Not Needed
- Animal research
- Case studies/Chart Reviews
- Surveys
To gain access to Florence, the research staff member requesting access must contact the Office of Clinical Research (OCR) at clinicalresearch@utrgv.edu. The following information must be provided (See UTRGV RA-206):
- Full name of staff member needing access
- Email address of staff member needing access
- Department of staff member needing access
- Role within the department
- How many studies does the staff member need to access? List the following for EACH study:
- Study PI
- Full Study Title
- Short Study Title/Study Number (if applicable)
- Role on Study
A representative from OCR will provide information and instructions on how to get started.
The Office of Clinical Research (OCR) has developed Standard Operating Procedures (SOPs) as guidance for any study team doing clinical research at UTRGV.
To access the complete list of Standard Operating Procedures (SOPs), visit the Guidelines & Procedures page.
For any questions, comments or concerns regarding the SOPs, please contact the OCR at clinicalresearch@utrgv.edu.
