Post Approval Monitoring (PAM)
Post Approval Monitoring
IACUC - IBC -IRB
The University of Texas Rio Grande Valley (UTRGV) Post Approval Monitoring (PAM) Program supports the institution's efforts to ensure that ethical and regulatory requirements are followed according to institutional policies and procedures and local, state, and federal regulations and guidelines. This program is designed to improve the quality of the research by ensuring congruency between what is described in the approved protocol and what occurs during the actual performance of research activities. The PAM Program has three pillars:
The purpose of the PAM is to promote research best practices on studies approved or under reliance agreement by the UTRGV:
- Institutional Animal Care and Use Committee (IACUC)
- Institutional Biosafety Committee (IBC)
- Institutional Review Board (IRB)
The program serves as an internal review process for proactively identifying and assessing potential problems and for developing and providing educational support and training to research personnel.
The PAM program aims to verify and document:
- the rights and well-being of research participants.
- the humane treatment of animals.
- safeguards for human health and the environment.
- availability of assistance for researchers to maintain or improve the quality and integrity of the research.
- compliance with institutional, local, state, and federal regulations and guidelines.
- congruence between approved protocol and research activities.
- the identification of resources for educational support to investigators and the research community.
For more information about Post-Approval Monitoring, please see Post-Approval Monitoring Standard Operating Procedure
Criteria for PAM Selection
All active approved protocols are eligible for PAM protocol review in one of the following selections:
- Routine - An annual random sample of a percentage of the active protocols will be selected, focusing on the criteria such as federally funded and regulated, high-risk studies, changes in research personnel, involving invasive technics, carryout in multiple sites, among others.
- Direct - Studies with non-compliance reports or concerns and/or designated by the institutional official.
- PI request - PI may opt to initiate a PAM protocol review to ensure compliance with federal and state regulations, institutional policies or as a proactive measure in anticipation of external monitoring or audits.
Overview of the Protocol Review Process
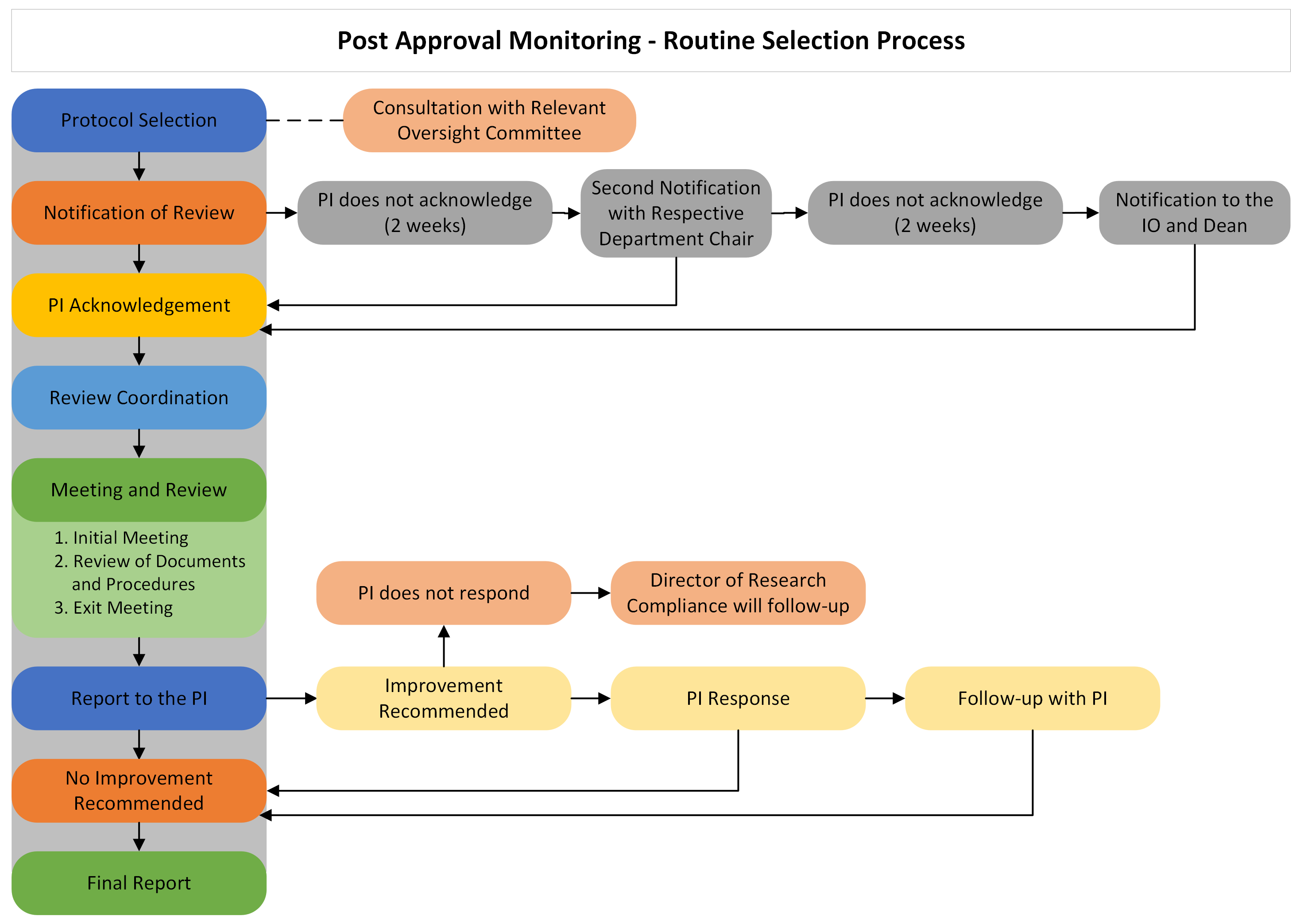
Protocols can be selected randomly or directed in response to concerns. Once selected, the Principal Investigator (PI) will be notified and will coordinate the visit with the PAM Monitor. The PAM Monitor will visit the laboratory or research facility and meet with the PI and/or research personnel. During the visit, the PAM Monitor may observe the research procedure, review documents, and interview the research team. After the visit, the PI will receive a report containing the findings from the PAM visit. The PI will be given an opportunity to make corrections by modifying techniques or submitting an amendment to their protocol(s). The PAM process will be considered complete only after all the necessary corrective actions have been taken care of.
